AP Chemistry 🧪
269 resourcesSee Units
Multiple Choice Practice for Atomic Structure and Properties
Welcome to Unit 1 AP Chemistry Multiple Choice Questions! Grab some paper and a pencil 📄 to record your answers as you go. You can see how you did on the Unit 1 Practice Questions Answers and Review sheet once you're done. Don't worry, we have tons of resources available if you get stumped 😕 on a question. And if solo study is not your thing, join a group in Hours!
Not ready to take a quiz yet? Take a look at the Intro to Unit 1.

Image courtesy of Pixabay
Facts about the test: The AP Chemistry exam has 60 multiple choice questions and you will be given 1 hour 30 minutes to complete the section. That means it should take you around 15 minutes to complete 10 questions.
*The following questions were not written by College Board and, although they cover information outlined in the AP Chemistry Course and Exam Description, the formatting on the exam may be different.
1. Which of the following isotopes has 30 neutrons?
(A) Manganese-55
(B) Nitrogen-15
(C) Phosphorus-30
(D) Zinc-65
2 .The ionization energy and atomic radius for aluminum is 578kJ/mol and 143 pm, respectively. What is the most likely ionization energy and atomic radius for silicon?
(A) 496 kJ/mol and 110 pm
(B) 496 kJ/mol and 180 pm
(C) 789 kJ/mol and 110 pm
(D) 789 kJ/mol and 180 pm
3. What is the electron configuration for Fe^2+?
(A) 1s^2 2s^2 2p^6 3s^2 3p^6 3d^6
(B) 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^4
(C) 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6
(D) 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^8
4. Given the following information, a 1.00 g sample of which of the following substances contains the greatest amount of chlorine?
(A) Li Cl
(B) K Cl
(C) Na Cl
(D) Rb Cl
5. Which of the following correctly identifies which has the greater first ionization energy, Na or Mg, and supplies the best reason?
(A) Mg, because of its greater nuclear charge.
(B) Mg, because it has a full sub-shell of electrons.
(C) Na, because it has a greater electronegativity
(D) Na, because of its greater mass.
6. Which of the following explains why the 1s peak for silicon is further to the left than the 1s peak for Carbon?
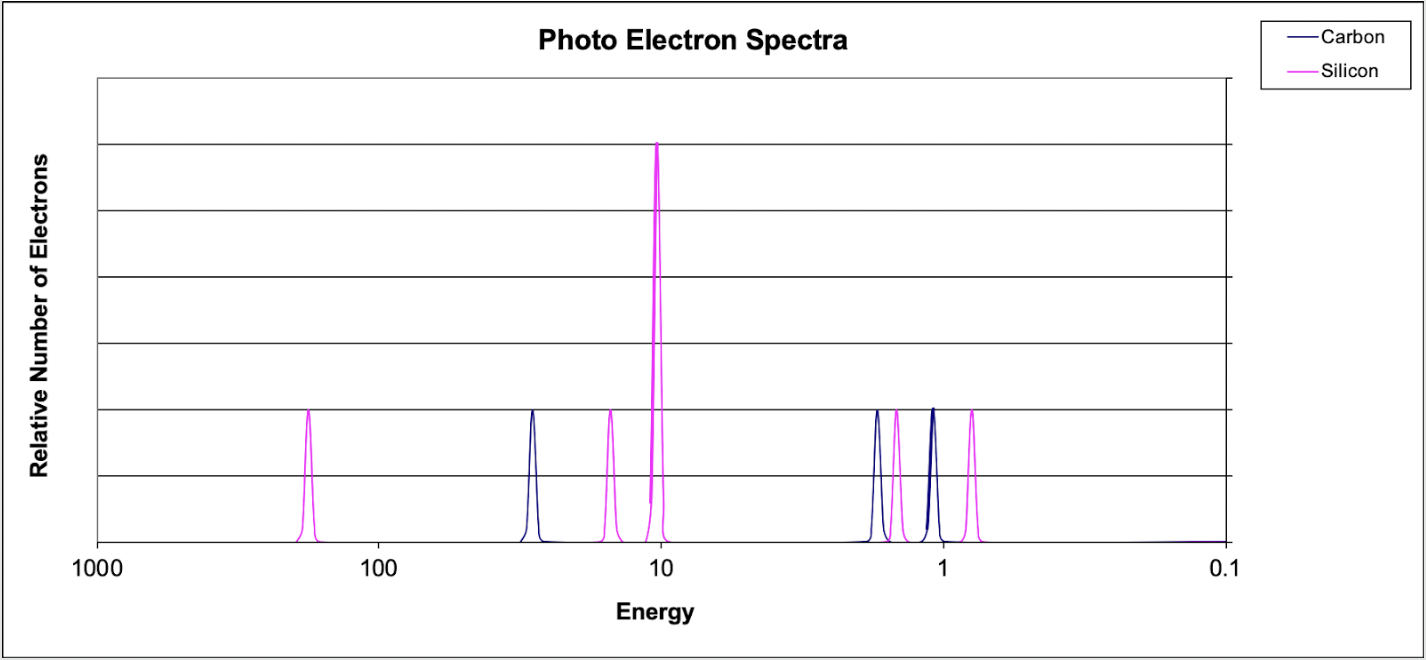
(A) Carbon has a greater electronegativity than silicon.
(B) Carbon has a smaller atomic radius.
(C) Silicon has a greater nuclear charge compared to carbon.
(D) Silicon's valence electrons occupy a larger energy level.
7. The successive ionization energies for an element are 899, 1757, 14848, and 21006 kJ/mol respectively. Which of the following correctly identifies the element and justifies this choice?
(A) Be, because it has two valence electrons.
(B) Be, because it has four valence electrons.
(C) O, because it has two valence electrons.
(D) O, because it has six valence electrons.
8. A compound contains only the elements C and H. If substance combusts and produces 44.0 g of CO_2 and 18.0 g of H_2O, then what is the empirical formula of the compound?
(A) C_2 H
(B) C H
(C) C H_2
(D) C H_4
9. Which of the following contains the greatest number of particles?
(A) 8 g of oxygen gas (O_2)
(B) 12 g of carbon (C)
(C) 22 g of carbon dioxide (CO_2)
(D) 110 g of lead (Pb)
10. A compound contains 24.0 g of carbon, 8.0 g of hydrogen, and 16.0 g of oxygen. What is the empirical formula of the substance?
(A) C_2 H_8 O
(B) C_4 H_8 O
(C) C_4 H_16 O_2
(D) C_24 H_8 O_16
11. Which of the following refers to the amount of energy required to remove an electron from an atom?
(A) Atomic Radius
(B) Electronegativity
(C) Electron Affinity
(D) Ionization Energy
12. Which of the following elements has the electron configuration described in the photo?
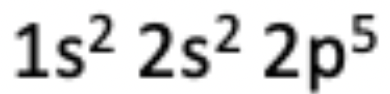
(A) Chlorine
(B) Fluorine
(C) Neon
(D) Nitrogen
13. Which of the following pairs are isotopes?
(A) C H_2 and C_2 H_4
(B) Chlorine-35 and Chlorine-37
(C) Oxygen-19 and Fluorine-19
(D) Water (H_2 O) and hydrogen peroxide (H_2 O_2)
14. Based on the data in the image, what element is represented?
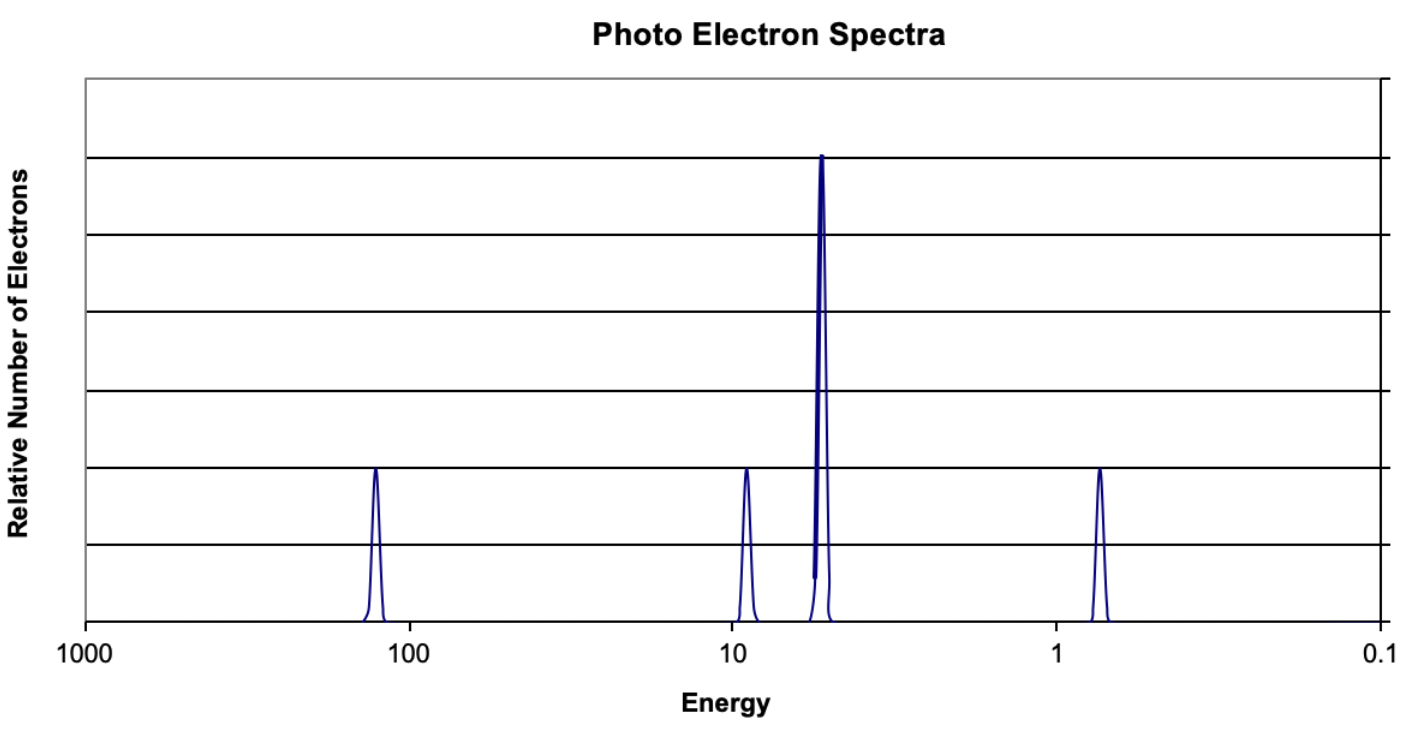
(A) Beryllium
(B) Carbon
(C) Magnesium
(D) Sodium
15. How many carbon atoms are in a 32.0 g sample of CH_4?
(A) 1.20 x 10^23 atoms of carbon
(B) 1.20 x 10^24 atoms of carbon
(C) 6.02 x 10^23 atoms of carbon
(D) 6.02 x 10^24 atoms of carbon
- 🙌 Time to check your answers on Unit 1 Practice Questions Answers and Review!
- 🤝Connect with other students studying AP Chem with Hours!
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

© 2023 Fiveable Inc. All rights reserved.