AP Chemistry 🧪
269 resourcesSee Units
Answers and Review for Multiple Choice Practice on Chemistry of Life
⛔STOP ! ⛔ Before you look at the answers, make sure you gave this practice quiz a try so you can assess your understanding of the concepts covered in Unit 1. Click here for the practice questions: AP Chemistry Unit 1 Multiple Choice Questions.

Image courtesy of Pixabay
Facts about the test: The AP Chemistry exam has 60 multiple choice questions and you will be given 1 hour 30 minutes to complete the section. That means it should take you around 15 minutes to complete 10 questions.
*The following questions were not written by College Board and although they cover information outlined in the AP Chemistry Course and Exam Description the formatting on the exam may be different.
1. Which of the following isotopes has 30 neutrons?
(A) Manganese-55
(B) Nitrogen-15
(C) Phosphorus-30
(D) Zinc-65
Answer: All of these isotopes are written with their mass number. The mass number is the number of protons plus neutrons. Manganese has 25 protons; therefore, 55-25 = 30 neutrons.
📄 Study AP Chemistry Unit 1.2 - Mass Spectroscopy of Elements
2 .The ionization energy and atomic radius for aluminum is 578kJ/mol and 143 pm, respectively. What is the most likely ionization energy and atomic radius for silicon?
(A) 496 kJ/mol and 110 pm
(B) 496 kJ/mol and 180 pm
(C) 789 kJ/mol and 110 pm
(D) 789 kJ/mol and 180 pm
Answer: When comparing elements in the same row, one must look at the number of protons the element has. Since silicon has more protons in its nucleus, it is better at attracting its electrons than aluminum is. This makes the first ionization energy greater and the radius smaller.
📄 Study AP Chemistry Unit 1.6 - Photoelectron Spectroscopy & Graph Interpretation
3. What is the electron configuration for Fe^2+?
(A) 1s^2 2s^2 2p^6 3s^2 3p^6 3d^6
(B) 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^4
(C) 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6
(D) 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^8
Answer: When electrons are removed from atoms, they are removed from the valence shell, which for iron is the fourth energy level. The valence electrons are the electrons in the highest energy level. Therefore, the electron configuration of Fe^2+ is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^6.
📄 Study AP Chemistry Unit 1.5 - Atomic Structure and Electron Configurations
4. Given the following information, a 1.00 g sample of which of the following substances contains the greatest amount of chlorine?
(A) Li Cl
(B) K Cl
(C) Na Cl
(D) Rb Cl
Answer: The percent composition of chlorine in LiCl is greatest, therefore, in samples of the same mass, the mass of chlorine will be greatest in the sample with the greatest mass percent.
📄 Study AP Chemistry Unit 1.1 - Moles and Molar Mass
5. Which of the following correctly identifies which has the greater first ionization energy, Na or Mg, and supplies the best reason?
(A) Mg, because of its greater nuclear charge.
(B) Mg, because it has a full sub-shell of electrons.
(C) Na, because it has a greater electronegativity
(D) Na, because of its greater mass.
Answer: When comparing two elements in the same period, one must consider the nuclear charge. The nuclear charge refers to the number of protons, of which magnesium contains more. with more protons, Mg has a greater force of attraction on its electrons.
📄 Study AP Chemistry Unit 1.6 - Photoelectron Spectroscopy & Graph Interpretation
6. Which of the following explains why the 1s peak for silicon is further to the left than the 1s peak for carbon?
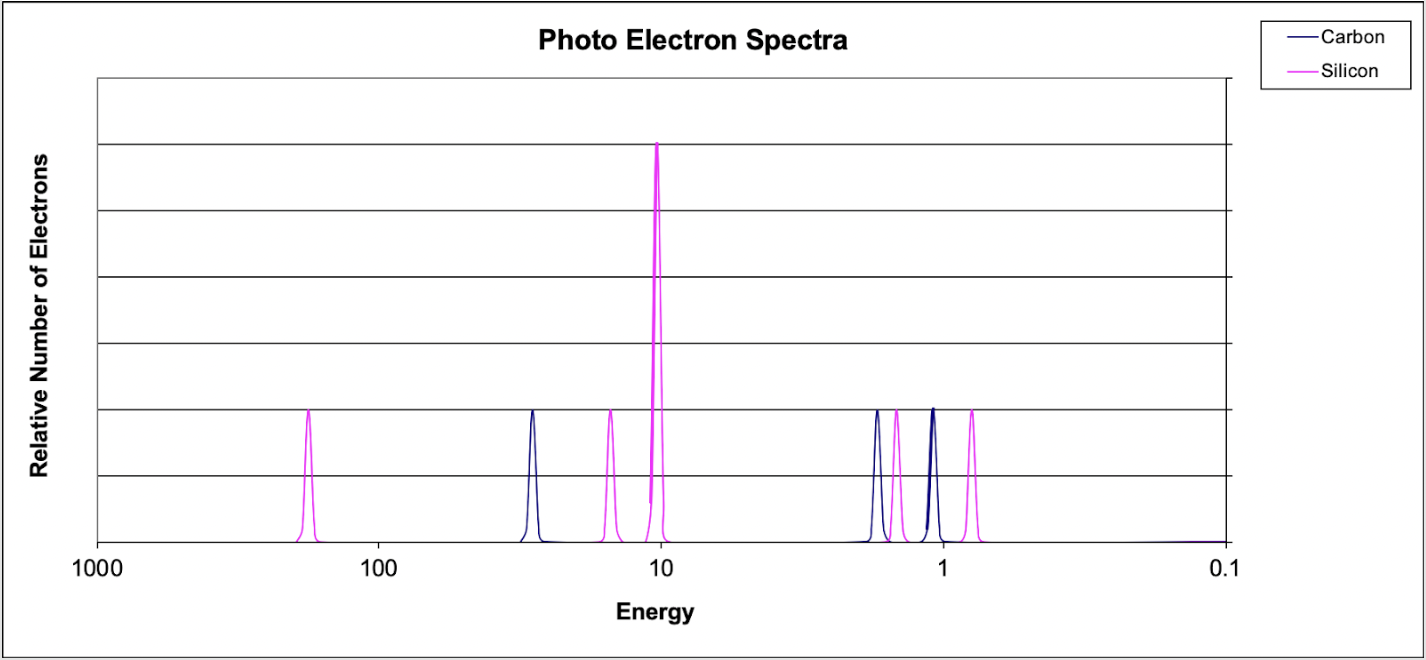
(A) Carbon has a greater electronegativity than silicon.
(B) Carbon has a smaller atomic radius.
(C) Silicon has a greater nuclear charge compared to carbon.
(D) Silicon's valence electrons occupy a larger energy level.
Answer: When comparing electrons in the same sub-level, one must compare the number of protons in the nucleus. More protons increases the nuclear charge and increases the attraction of a respective sub-level.
📄 Study AP Chemistry Unit 1.6 - Photoelectron Spectroscopy & Graph Interpretation
7. The successive ionization energies for an element are 899, 1757, 14848, and 21006 kJ/mol respectively. Which of the following correctly identifies the element and justifies this choice?
(A) Be, because it has two valence electrons.
(B) Be, because it has four valence electrons.
(C) O, because it has two valence electrons.
(D) O, because it has six valence electrons.
Answer: The number of valence electrons is indicated by the large increase in the ionization energy. The jump occurs between the second ionization and the third, indicating that there are two valence electrons.
📄 Study AP Chemistry Unit 1.8 - Valence Electrons and Ionic Compounds
8. A compound contains only the elements C and H. If substance combusts and produces 44.0 g of CO_2 and 18.0 g of H_2O, then what is the empirical formula of the compound?
(A) C_2 H
(B) C H
(C) C H_2
(D) C H_4
Answer: Carbon and hydrogen are in a 1mol:2mol ratio respectively.
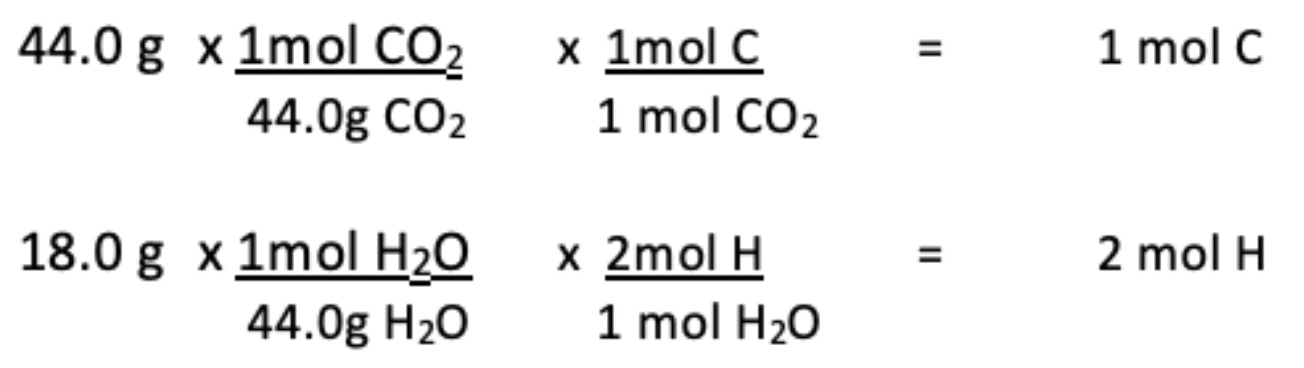
📄 Study AP Chemistry Unit 1.1 - Moles and Molar Mass
9. Which of the following contains the greatest number of particles?
(A) 8 g of oxygen gas (O_2)
(B) 12 g of carbon (C)
(C) 22 g of carbon dioxide (CO_2)
(D) 110 g of lead (Pb)
Answer: Since the molar mass of Carbon is ~12 g/mol, this is the only sample that contains roughly 1 mol of particles. All the other values contain less than one mole.
📄 Study AP Chemistry Unit 1.1 - Moles and Molar Mass
10. A compound contains 24.0 g of carbon, 8.0 g of hydrogen, and 16.0 g of oxygen. What is the empirical formula of the substance?
(A) C_2 H_8 O
(B) C_4 H_8 O
(C) C_4 H_16 O_2
(D) C_24 H_8 O_16
Answer: Carbon, hydrogen, and oxygen are in a 2mol C : 8mol H : 1mol O ratio
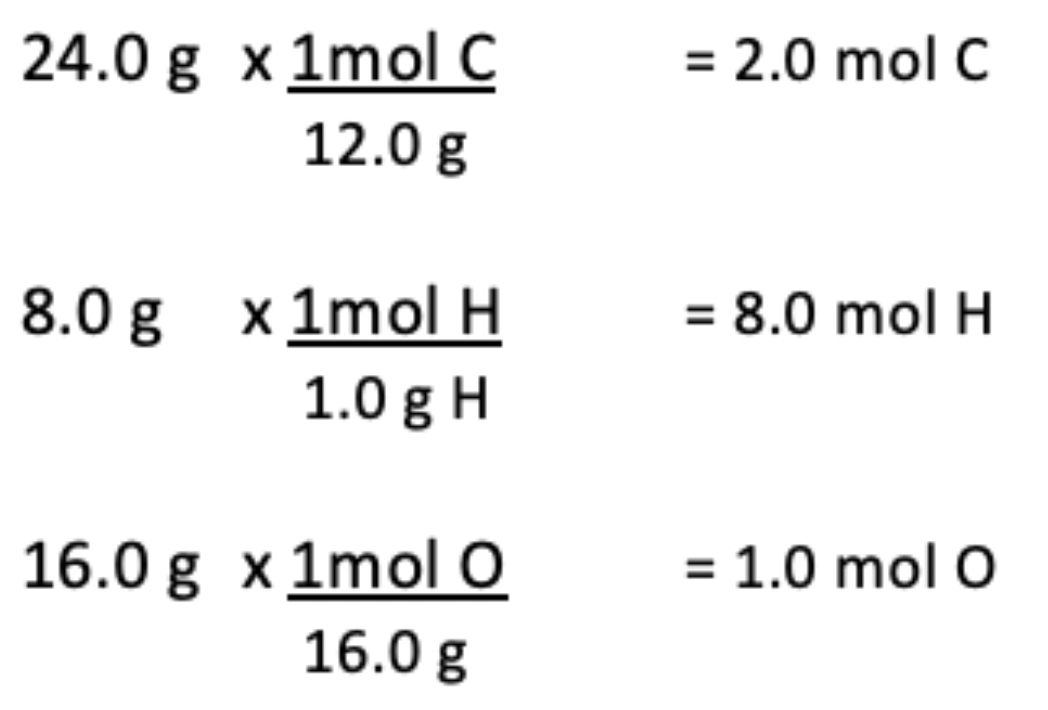
📄 Study AP Chemistry Unit 1.1 - Moles and Molar Mass
11. Which of the following refers to the amount of energy required to remove an electron from an atom?
(A) Atomic Radius
(B) Electronegativity
(C) Electron Affinity
(D) Ionization Energy
Answer: Ionization energy is defined as the amount of energy required to remove an electron from an atom.
📄 Study AP Chemistry Unit 1.6 - Photoelectron Spectroscopy & Graph Interpretation
12. Which of the following elements has the electron configuration described in the photo?
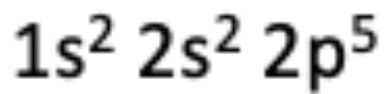
(A) Chlorine
(B) Fluorine
(C) Neon
(D) Nitrogen
Answer: Fluorine has 9 electrons that are defined by the electron configuration in the image.
📄 Study AP Chemistry Unit 1.5 - Atomic Structure and Electron Configurations
13. Which of the following pairs are isotopes?
(A) C H_2 and C_2 H_4
(B) Chlorine-35 and Chlorine-37
(C) Oxygen-19 and Fluorine-19
(D) Water (H_2 O) and hydrogen peroxide (H_2 O_2)
Answer: Isotopes are defined as elements of the same element with different numbers of neutrons.
📄 Study AP Chemistry Unit 1.2 - Mass Spectroscopy of Elements
14. Based on the data in the image, what element is represented?
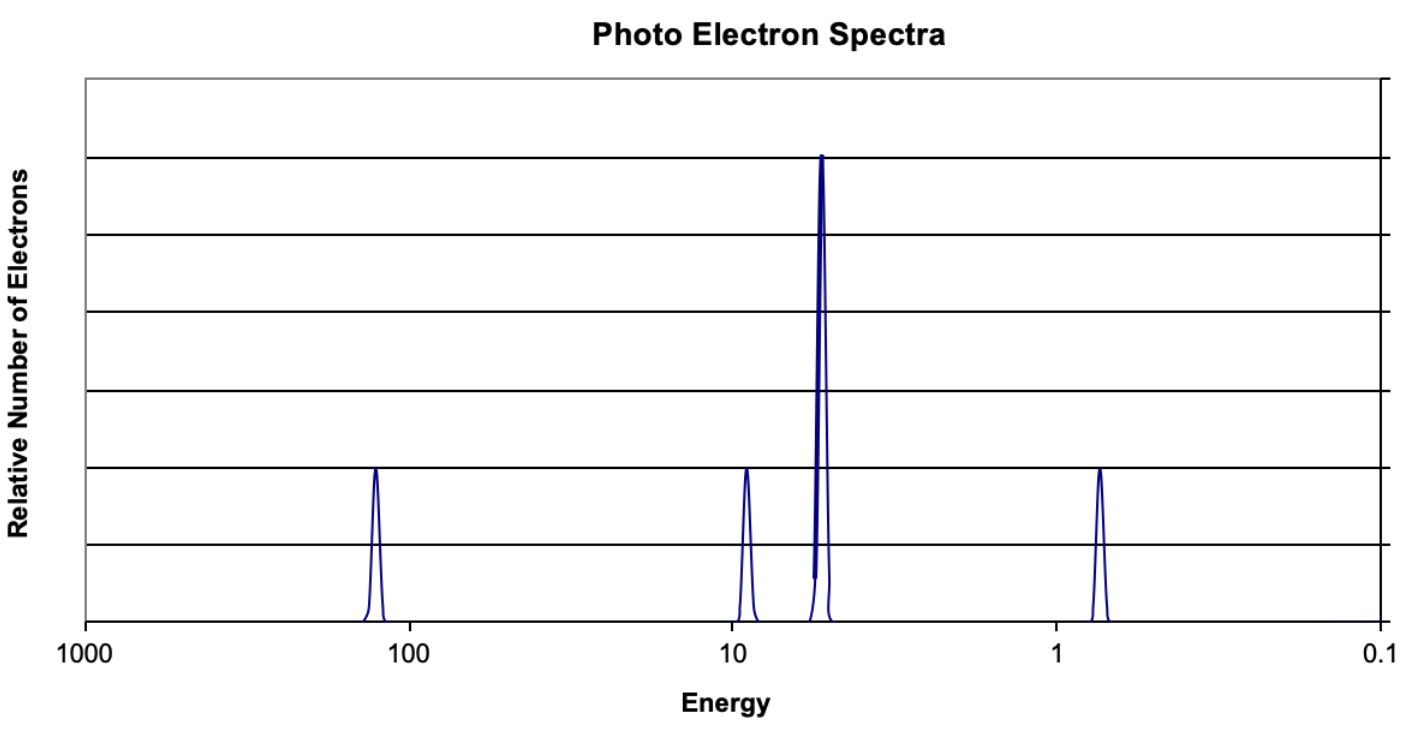
(A) Beryllium
(B) Carbon
(C) Magnesium
(D) Sodium
Answer: Magnesium has the electron configuration of 1s^2 2s^2 2p^6 3s^2. This is represented by the photo electron spectrum.
📄 Study AP Chemistry Unit 1.5 - Atomic Structure and Electron Configurations
15. How many carbon atoms are in a 32.0 g sample of CH_4?
(A) 1.20 x 10^23 atoms of carbon
(B) 1.20 x 10^24 atoms of carbon
(C) 6.02 x 10^23 atoms of carbon
(D) 6.02 x 10^24 atoms of carbon
Answer:
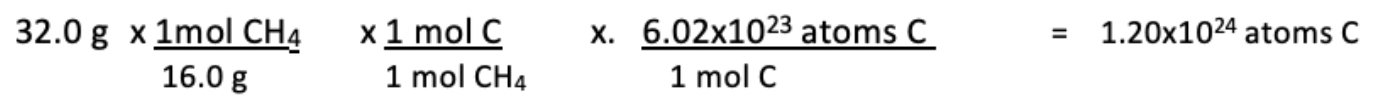
📄 Study AP Chemistry Unit 1.6 - Photoelectron Spectroscopy & Graph Interpretation
What can we help you do now?
- 🔍Check out all of the resources for AP Chem Unit 2
- 🦘Jump to AP Chem Unit 2 Multiple Choice Questions
- 🤝Connect with other students studying AP Chem with Hours
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

© 2023 Fiveable Inc. All rights reserved.