7.9 Introduction to Le Châtelier’s Principle
6 min read•january 29, 2023
Dalia Savy
Dylan Black
Dalia Savy
Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
Equilibrium thus far has considered only reactions going straight to equilibrium and then staying there. However, it doesn't have to be that way. We can actually control what happens in a chemical system...👀
What if we wanted to say, shift an equilibrium towards the products or towards the reactants? How does equilibrium shift based on certain stressors that are added to a system? This section will address that very question by introducing a new rule: Le Châtelier’s Principle.
What Is Le Châtelier’s Principle?
Le Châtelier’s Principle states one key rule about how a system in equilibrium will react to an external pressure: “if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to counteract the change to reestablish an equilibrium” (LibreTexts). Let’s break down each part of this.
Dynamic Equilibrium and Stressors
We begin by establishing that we are in a dynamic equilibrium. This just means that we have some reaction that is at equilibrium. We call it dynamic equilibrium because equilibrium does not mean the reaction has stopped but rather that the concentrations of reactants and products have not changed. You may be wondering what other type of equilibrium there is. There is also something called static equilibrium and it's basically a state of balance in which the system is not moving. No need to worry about that, try to focus on dynamic equilibrium!
Next, our dynamic equilibrium is disturbed by “changing the conditions”. We’ll cover some specific disturbances in the next section, but generally, this phrase refers to any external change that comes to the system from outside. This could mean adding more of something, increasing temperature, changing pressures, etc. Remember when we discussed how external factors can change the kinetics of a reaction? Well...they can affect where a reaction stands with regard to equilibrium.
Finally, and this is the part where Le Châtelier’s Principle answers our question, the equilibrium will shift to counteract the change and re-establish equilibrium. Think of Le Châtelier’s principle as a seesaw that always wants to remain in balance. If you add a 50-pound weight to one side, Le Châtelier’s principle will respond by counteracting that change and shifting some of that 50lbs to the other side.
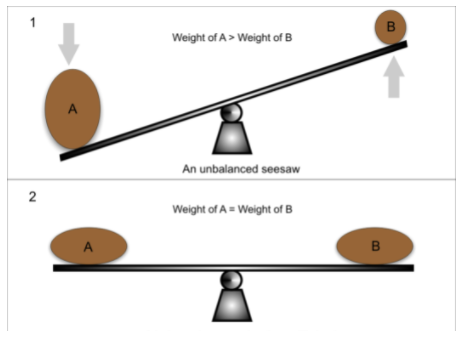
Image Courtesy of Greater Minds Tutors
Factors That Influence Equilibrium
There are several factors that can influence equilibrium in a chemical or physical system and you should understand how Le Châtelier’s applies.
Concentration
Changes in concentration are the clearest way in which equilibrium can shift. The concentrations of the reactants and products are constant at equilibrium, so what happens if external stress adds or removes substances? Note that the K value of the reaction is not going to change, just the concentrations at equilibrium. This is referred to as inducing new stress to the system, and based on Le Châtelier’s Principle, we can figure out which direction the reaction will shift to re-establish equilibrium.
If we add more reactants to the system, the system will respond by counteracting that change and creating more products to return to equilibrium. Similarly, if we add products to a system the system will respond and create more reactants. Changes in concentration as stressors are pretty easy to compensate, as you can consume the substances there are more of and create the substances there are less of.
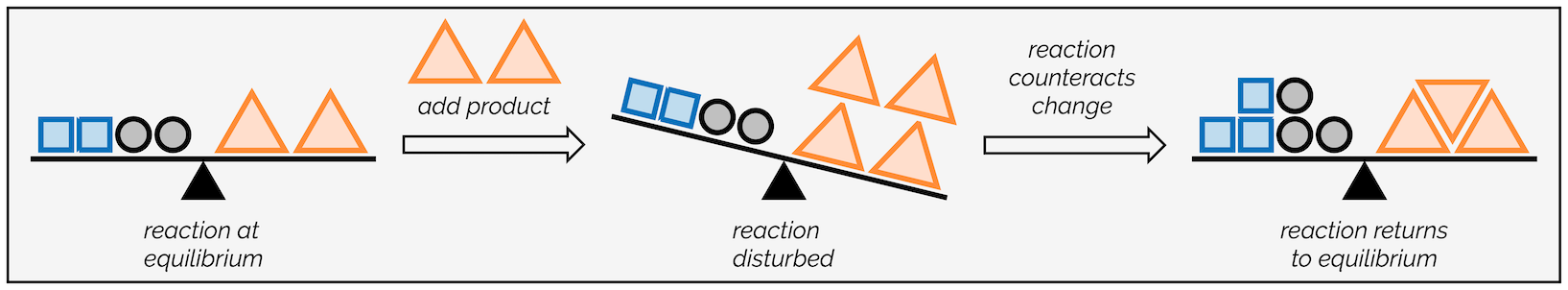
Image Courtesy of Making Molecules
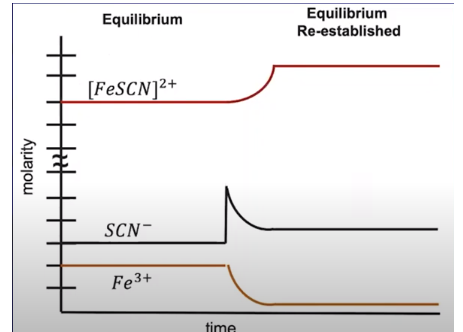
Let's consider the following chemical equation: Fe³⁺ + SCN⁻ ⇌ FeSCN²⁺. Imagine the system is at equilibrium, and we add a compound containing the SCN⁻ ion to the system, increasing its concentration. That excess ion is going to be consumed to create more products and re-establish the equilibrium. We can see this in the graph below.
Notice that there is a sharp spike in the concentration of SCN⁻, indicating that its addition is the stress the system responds to. Once the [SCN⁻] is added, we see a decrease in [Fe³⁺] and [SCN⁻] as responses to the stress: the system responds by creating more [FeSCN]²⁺.
Another important part of how concentration impacts equilibrium is adding a compound that will react with a reactant or product to take it out of the system. For example, adding a compound containing hydroxide will cause the hydroxide to react to form Fe(OH)₃, taking the Fe³⁺ ion out of the system. Based on Le Châtelier’s principle, the system will respond by increasing the amount of Fe³⁺ and SCN⁻ present in the system and decreasing the concentration of [FeSCN]²⁺.
Temperature
Temperature changes, like changes in concentration, have an impact on equilibrium. In this case, the change in equilibrium depends on whether or not our reaction is exothermic (heat releasing) or endothermic (heat absorbing). Remember from unit six that we can identify whether a reaction is exothermic or endothermic by observing its change in standard enthalpy, or ΔH°.
If ΔH° < 0, or negative, our reaction is exothermic, and if ΔH° > 0, or positive, our reaction is endothermic. When referring to Le Châtelier’s Principle, we can think of heat as either a reactant or product and then refer to concentration for similar conclusions for temperature.
For example, consider the reaction N₂ + 3H₂ ⇌ 2NH₃. This reaction has a ΔH° of -92 kJ/mol. Since ΔH° is negative, our reaction is exothermic and heat is released into the surroundings, implying that heat can be thought of as a product. This means that if the temperature increases, our equilibrium will shift to produce more reactants. We're basically fueling the reaction that takes in heat, which in this case, is the reverse reaction.
The opposite would occur if the reaction was endothermic and the temperature was a reactant. With temperature, try to think about which direction of the reaction you are "fueling;" that is the direction the reaction will shift.
Pressure
When dealing with a system involving gases, changes in pressure (and therefore the volume, remember Boyle’s Law!) will change concentrations of species in the system.
In general, if the pressure on a system increases or the volume decreases, the equilibrium will shift towards the side with fewer moles of gas and vice versa. If there is no difference in moles of gas, there will be no change either way.
When we talk about moles of gas, we’re talking about the coefficients in the reaction.
For example, consider the reaction A + B ⇌ 2C + 3D where A B C and D are gasses. On the left, there are 2 total moles of gas because 1 + 1 = 2. On the right, there are 5 total moles because 2 + 3 = 5. Therefore, if the pressure were to increase on the system, the equilibrium would shift left and products would be converted into more reactants.
However, there is one caveat to this rule that has to do with inert gases. An inert gas is a gas that does not react when added to a mixture of chemicals. If an inert gas is added or removed from a vessel, there is no impact on equilibrium. For example, if Helium gas was pumped in, there would be no impact as far as equilibrium is concerned.
Summary of Le Châtelier’s Principle
We just went over so many rules, so here is a nice table that can break them down a bit:
| Stress | Shift | Explanation |
| Increase the concentration of a substance | Away from the substance | Extra concentration needs to be used up |
| Decrease the concentration of a substance | Towards the substance | Need to produce more of the substance that was removed to make up for the loss |
| Increase the pressure of the system | Toward fewer moles of gas | Boyle's law: pressure increase = volume decrease |
| Decrease the pressure of the system | Toward more moles of gas | Boyle's law: pressure decrease = volume increase |
| Increase the temperature of the system | Away from the heat, favoring the endothermic reaction | Extra heat has to be used up to fuel the reaction |
| Decrease the temperature of the system | Toward the heat, favoring the exothermic reaction | More heat needs to be produced to make up for the loss |
| Adding a catalyst | --no shift-- | The rates of both the forward and reverse reactions are increased by the same amount. Adding a catalyst only affects kinetics, not equilibrium. |
Table Courtesy of Socratic
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

© 2023 Fiveable Inc. All rights reserved.