Dalia Savy
Kanya Shah
Dalia Savy
Kanya Shah
AP Chemistry 🧪
269 resourcesSee Units
So far, we learned about intramolecular forces within ionic solids, metallic solids, and alloys in unit two. Now that we've discussed the different types of intermolecular forces, let's discuss more solids!
The structures and properties of solids can be classified according to the forces that hold the atoms together.
- Ionic solids are held together by electrostatic forces between cations and anions (think Coulomb's law).
- Metallic solids are held together by metallic bonds between atoms.
- Covalent network solids are held together by an extended network of covalent bonds (think of diamonds💎).
- Molecular solids are held together by weak IMFs.
Now that the basics are out of the way, let’s dig into the details!
Amorphous Solids
Amorphous solids are those that do not have a long-range, periodic crystal structure. They have considerable disorders in their structures and it is all due to cooling quickly😰.
These are their own category and will not be studied in-depth in AP Chemistry. However, some examples of amorphous solids include gum, glass, and rubber.
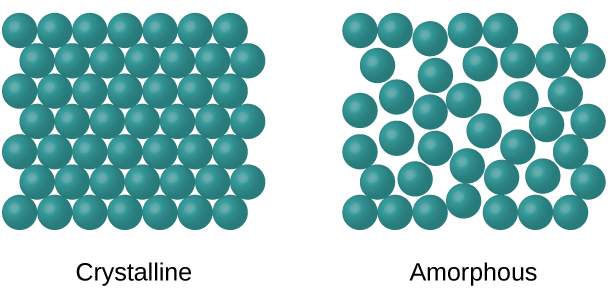
Image Courtesy of OpenTextBC
Crystalline Solids
Crystalline solids are the greater category of solids and include all of the different types of solids that the College Board wants you to know for the AP Chemistry exam: ionic solids, covalent network solids, molecular solids, and metallic solids.
In crystalline solids, particles are arranged in a regularly repeating pattern. Crystalline solids have a definite melting point, and they tend to be more ductile and less brittle than amorphous solids.
Structure of Crystalline Solids
The geometrical pattern of points on which crystalline solids are arranged is called a crystal lattice. The basic building block, or smallest repeating unit, that makes up a crystal lattice is a unit cell.
In other words, the unit cell is the smallest unit that can be used to describe the crystal lattice, and it is repeated throughout the solid to form the crystal lattice.
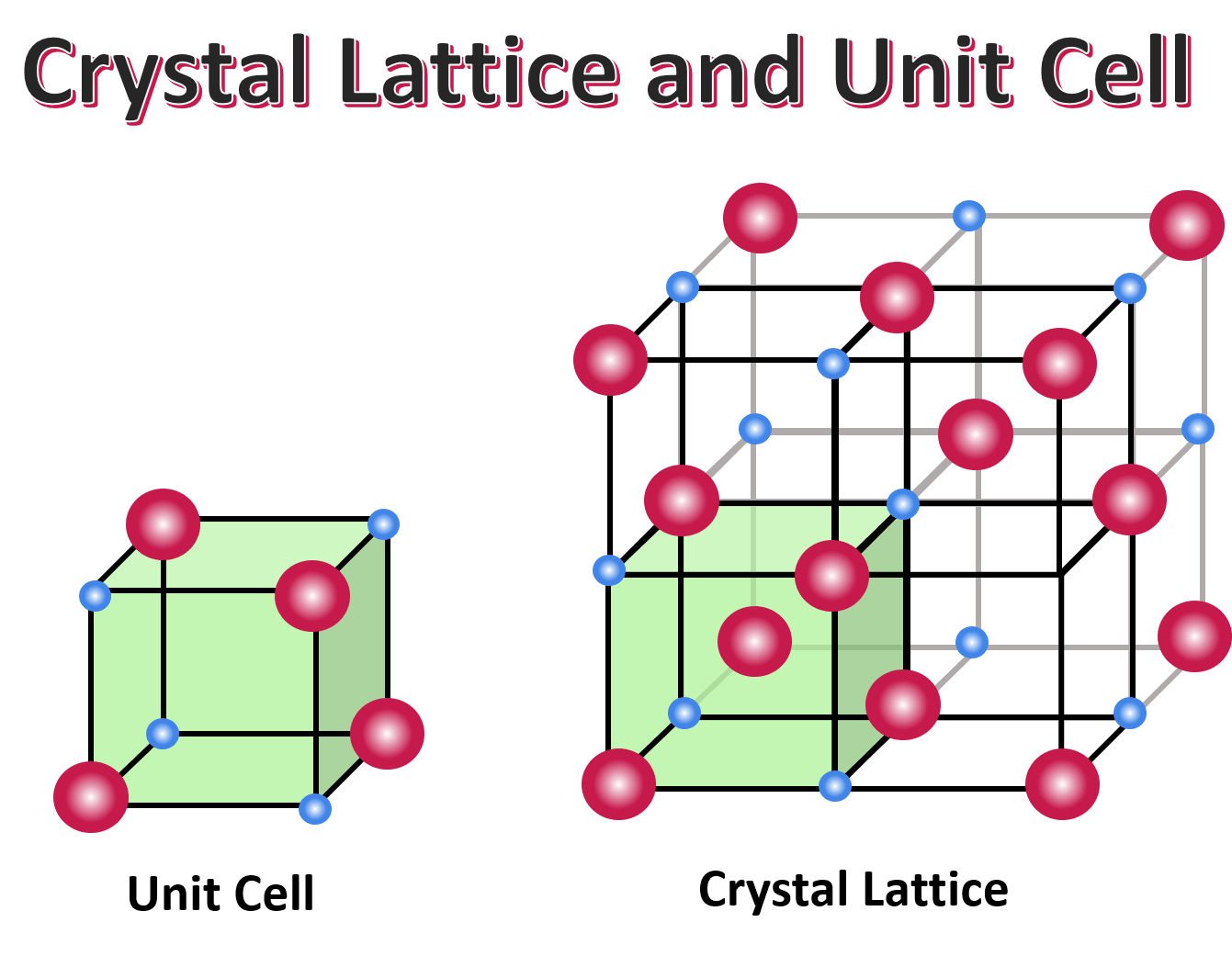
Image Courtesy of Expii; You do not need to know the types of unit cells, but here is an overview of what this term really means!
Basically, if one cube represents a unit cell, then 100 cubes combined into a structure would represent a crystal lattice.
Metallic Solids
Metallic solids are composed of metal atoms held together by metallic bonds.
When metals ionize, they lose a valence electron and become positive ions or cations. Metallic bonding can therefore be represented as an array of cations surrounded by this "sea" of valence electrons. The valence electrons are delocalized and visualized as being free to move throughout the entire metal.
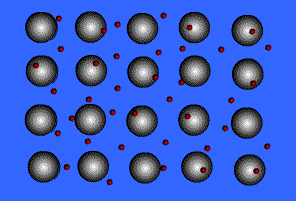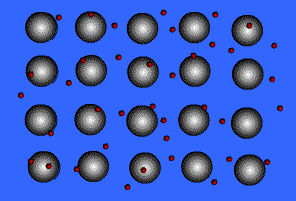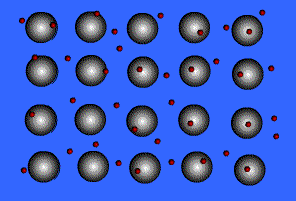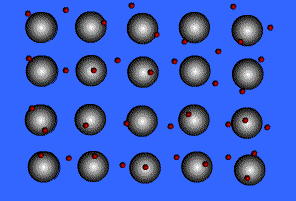
Animation Courtesy of DocSity
Because of the delocalization of valence electrons, metallic substances have very unique properties:
- Good conductors of electricity ⚡
- High melting and boiling points 🌡️
- Shiny appearances 🌟
- Malleability and ductility 🔌
Alloys
Alloys are materials that possess characteristic metallic properties and are composed of two or more elements. The elements in an alloy can be distributed as either homogeneous or heterogeneous.
- In a substitutional alloy, the atoms of the minority element occupy positions normally occupied by atoms of the majority element.
- In an interstitial alloy, atoms of the minority elements, often smaller nonmetallic atoms, occupy interstitial positions that lie in the “holes” between atoms of the majority element.
Image Courtesy of the University of North Florida
Ionic Solids
Ionic solids consist of cations and anions held together in a crystal lattice by electrostatic forces. Since these interactions are quite strong, ionic compounds tend to have a higher melting point.
The key trend to note is that the attractions become stronger as the charges of ions increase and/or the sizes of the ions decrease (Coulomb's law anyone?).
The presence of both attractive and repulsive interactions helps to explain why ionic compounds are brittle. Ionic solids are characterized by a crystal lattice of ions attracted to each other.
They only conduct electricity when ions are mobile and can flow. This occurs when ionic solids are melted or put into a solution.
Something new to note about ionic solids is that they possess ions at the lattice points of the solid structure.
Molecular Solids
Molecular solids consist of atoms or molecules held together by IMFs. They possess strong intramolecular forces (covalent bonds), but weak intermolecular forces.
Let's break down the general properties of molecular solids:
- 🌡️Low melting and boiling points - Because the intermolecular forces holding molecular solids together are weak, molecular solids have relatively low melting and boiling points.
- The melting point depends on the strength of the IMFs, as well as the efficiency with which the molecules can pack together. Two good examples of molecular solids are ice and sucrose.
- 💎Brittle and hard - Molecular solids tend to be brittle and hard, as they are held together by weak intermolecular forces that are easily broken.
- 🚫🔌Poor conductors of heat and electricity - Molecular solids are poor conductors of heat and electricity, as they do not have a metallic structure with free electrons that can easily flow through the solid. Their valence electrons are rather tightly held within covalent bonds.
Covalent Network Solids
Covalent network solids consist of atoms held together in large networks by covalent bonds. These solids are much harder and have higher melting points than molecular solids. Important examples to keep in mind are graphite and diamond, which are both made up of carbon.
These large networks could be atoms bonded in a 3D network, like diamonds, or 2D layers, like graphite.
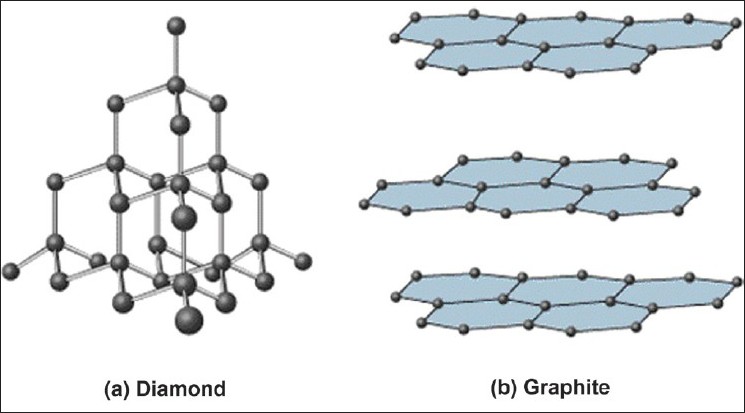
Image Courtesy of JidOnline
Graphite
- Layers of Carbon atoms arranged in rings
- sp2 hybridized, pi bonds
- delocalized electrons --> good conductor of heat and electricity
- strong bonds within the layers, but weak bonds between the layers
- soft because the layers can slide past each other (due to weak bonds holding the layers together)
Diamond
- hardest naturally occurring substance
- sp3 hybridized
- insulator
Comparing Solids
When comparing properties among the different solids, remember this chart:
| Type of Solid | Form of Unit Particles | Forces Between Particles | Properties | Examples |
| Molecular🧊 | Atoms or Molecules | LDFs, dipole-dipole, hydrogen bonding | fairly soft, low melting point, bad conductor | Argon, methane, sucrose, dry ice |
| Covalent Network💎 | Atoms connected in a network of covalent bonds | Covalent Bonds | Very hard, very high melting point, bad conductor | diamond, quartz |
| Ionic🧂 | Positive and Negative Ions | Electrostatic attractions | Hard and brittle, high melting point, bad conductor | salts (NaCl) |
| Metallic✨ | Atoms | Metallic Bonds | Varying hardness and melting points, good conductor, malleable, ductile | metals! Cu, Fe, Al |
Table Courtesy of Unknown Source
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

© 2023 Fiveable Inc. All rights reserved.