Dalia Savy
A
Anika P
Dalia Savy
A
Anika P
AP Chemistry 🧪
269 resourcesSee Units
Atoms come together to make molecules, right? What terms would you use for the two specific atoms that make up an ionic solid? The words you use can make or break your FRQ response, so make sure you are careful!
Ionic solids are made up of a positive cation and a negative anion. Ionic bonding typically occurs when a metal's valence electron is transferred to a nonmetal. Since the metal loses a negative electron, it becomes a cation. On the other hand, since the nonmetal gains a negative electron, it becomes an anion.
The positive cation and negative anion interact because of their opposite charges, but how much they interact depends, again, on Coulomb's Law.
Structure of Ionic Solids
As mentioned earlier, ionic interactions can produce brittle, hard solids that have high melting points. This is due to the ions being held in a 3-D array known as a crystal lattice.
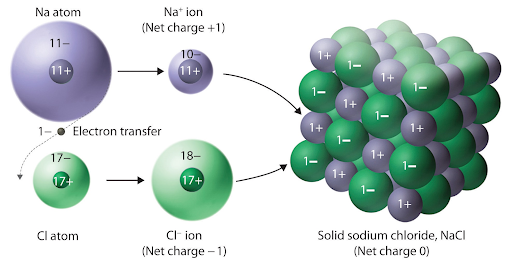
The reason for this is that ions are attracted to their opposites, and so negative ions surround positive ones and vice versa. You can think of this arrangement as a way to maximize the attractive forces between the cations and anions while minimizing the repulsive forces.
Another characteristic of this arrangement is how the sizes of the ions generally fit well together. When metals ionize, they lose an electron, making them decrease in size. When nonmetals ionize, they gain an electron, making them increase in size. You could see this periodic trend above with a sodium and chlorine atom ionizing. This allows the small cations (Na+ ions) to fit between the larger anions (Cl- ions). This does not happen for all ionic solids, but it is something to note as it impacts the forces experienced.
Representation of Ionic Solids
It is important to note that particle diagrams for ionic substances look different from that of molecular substances (that have covalent bonds). Covalent substances are usually represented by a molecule (such as H2O) while ionic substances are represented by a network of positive😊 and negative😞 ions.
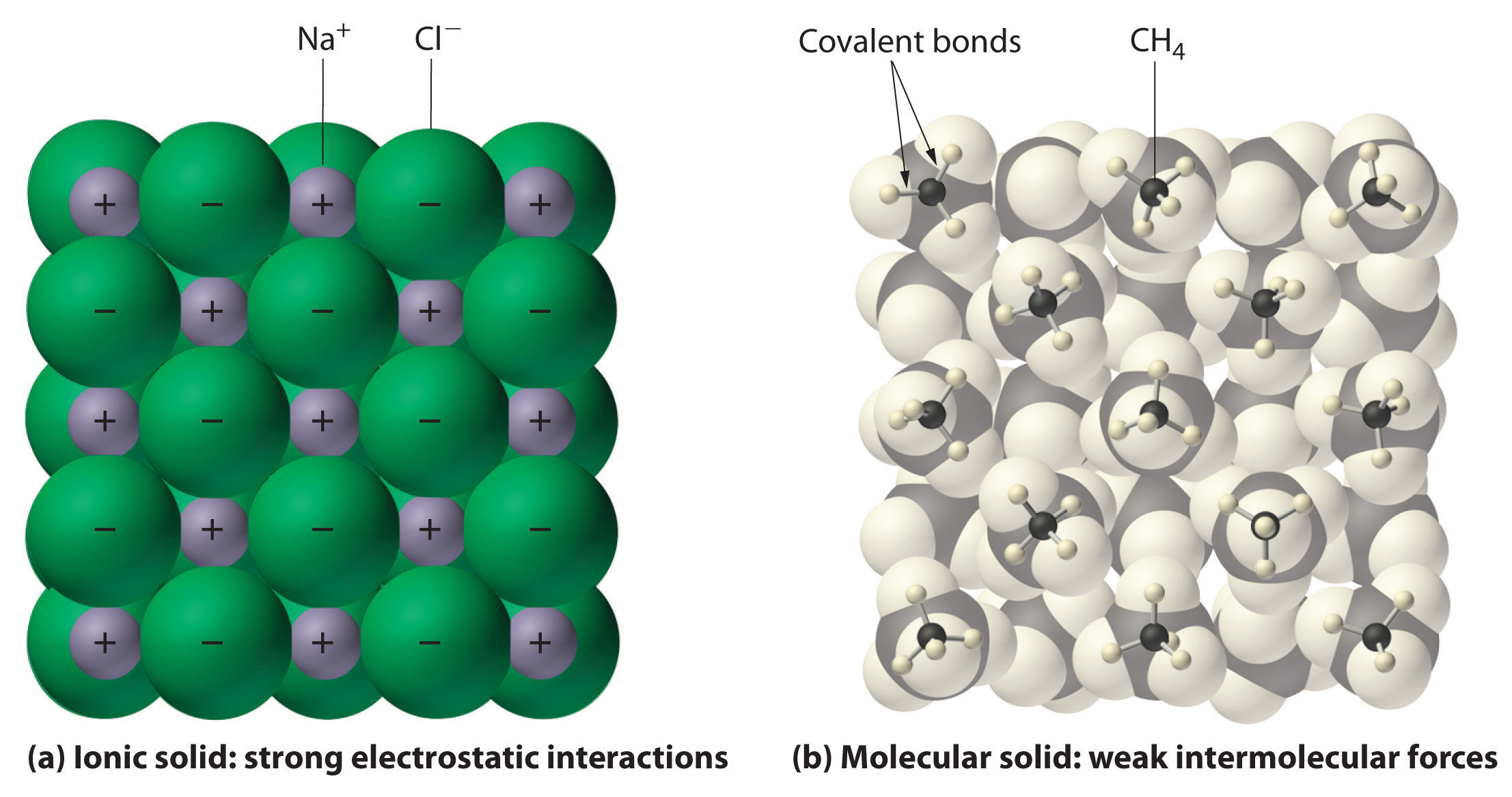
Image Courtesy of Principles of General Chemistry; We'll go into this further, but it is good to understand the difference between the two.
Explaining the Lattice Structure
The lattice structure can be explained by the strong electrostatic forces that arise between cations and anions because of their opposing charges. Coulomb's law describes these exact forces, telling us that the electrostatic force between a cation and an anion is directly proportional to their charges and inversely proportional to the distance between them.
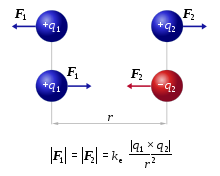
Image Courtesy of Wikipedia
It is rather important to understand that the strength of these electrostatic forces depends on two factors:
- Magnitude of charge - The greater the charge of the cation and anion, the stronger the attraction between the two.
- Distance between the nuclei of the ions - The closer the cation and anion, the stronger the attraction between the two.
This is why size is great to note! If the small sodium cations fit between the chloride anions, there is a smaller distance between their nuclei. According to Coulomb's law, this increases the strength of attraction between the sodium and chloride ions!
Remember, attractive forces between cations and anions are maximized in the lattice structure while repulsive forces are minimized.
Properties of Ionic Substances
Ionic substances are typically solids at room temperature and are known for their very high melting and boiling points. Let's break down the general properties of ionic substances:
- 🌡️ High melting and boiling points - The strong electrostatic forces that make up an ionic solid require a lot of energy to overcome, which is why it takes a lot of heat to melt or boil an ionic substance.
- 🚫🔌Poor conductors of electricity as solids - In a lattice, electrons are stuck in place, or in other words, they are localized. Since there are no electrons moving around and carrying an electric current, ionic solids are generally poor conductors of heat and electricity.
- ⚡Good conductors of electricity in their liquid or aqueous states - When ionic solids melt into liquids, their ions are able to move around the liquid phase. In other words, the delocalization of electrons allows them to conduct heat and electricity. This applies to the aqueous phase as well.
- 💎 Hard and brittle - Ionic solids typically have these characteristics because of their strong electrostatic forces. These forces make the ionic solids very difficult to deform.
Lattice Energy
Lattice Energy is the energy released when ions bond to form an ionic solid. We're back to Coulomb's law! It actually relates to lattice energy too; it's kind of everywhere.
Review of Coulomb's Law
Let's ease into lattice energy...do you also remember how to find out which ionic compound would have a higher melting point? Let's review that, it'll help with lattice energy I promise!
- Out of NaF and NaCl, which one has the higher melting point?First, we would look at the charges of the ions. Here, they are both +1/-1, so charges can't have an effect on the differences in melting point. Then, we would look at which ions are smaller. Remembering the periodic trends, F- is much smaller than Cl-. Since it is smaller, it can be closer to the Na+ ion and increase the strength of attraction. Therefore, it takes more energy to break the bond, increasing the melting point.
If you still don't remember that process, you can review here!
Putting Everything Together
Lattice energy depends on the same two concepts that you used in that question: charge and distance. Coulomb's Law directly relates to melting point and lattice energy so just remember:
The smaller the size and the higher the charge, the higher the lattice energy🤔. Therefore, the higher the melting point of an ionic solid, the higher the lattice energy.
Easy rule, right? Let's try a few out:
Which of the following compounds has a higher lattice energy?
- NaF or NaCl
- Since we already thought this question through, it's easy to tell that NaF has the higher lattice energy.
- MgO or NaF
- Charge first: Mg and O have a +2/-2 charge, while Na and F have a +1/-1 charge. You don't even have to check the distance, MgO must have the higher lattice energy.
- NaF or KCl
- Since these ions are in the same groups, they have the same charge. We must remember the periodic trends and that the farther down in a group you go, the larger the ions get. Therefore, K+ and Cl- are larger than Na+ and F-. With the smaller size, NaF has a higher lattice energy.
- LiCl or NaCl
- Last one! Since Li+ is smaller, LiCl must have a higher lattice energy.
Check your Understanding
The following question is from the Advanced Placement YT Channel. All credit to them.
Answer the following questions related to Mg and Sr.
- Write the complete ground state configuration for the ions Mg+2 and Sr+2.
- Do you predict that the ionic radius of Sr+2 is larger or smaller in size than the ionic radius of Mg+2? Justify your answer in terms of atomic structure and the electron configuration of each ion.
- The lattice energy of MgCl2(s) is equal to 2300 kJ/mol. Do you predict that the lattice energy of SrCl2(s) should be less than or greater than 2300 kJ/mol? Justify your answer in terms of Coulomb's law.
Sample Responses
(1) Looking at the periodic table and remembering electron configuration, you should get:
You cannot leave the electron configurations of Mg and Sr as your final answer! Make sure you always answer what they are asking.
I originally wrote down the electron configurations of Mg and Sr and then took off two valence electrons to get the final electron configurations of cations Mg+2 and Sr+2.
(2) This question goes back to periodic trends. Which ion has more electrons and electron shells? Sr2+ does, so it has a larger ionic radius.
Sample Response: Sr+2 has a larger ionic radius than Mg+2 because it has more occupied electron shells. The valence electrons in Sr2+ are in the 4th energy level whereas the valence electrons in Mg2+ are in the 2nd energy level. Electrons in the 4th energy level are generally farther away from the nucleus, making the ion larger.
(3) Charges are the same, so size must be accountable for the difference in lattice energy.
Sample Response: Coulomb's law states that the higher the charges of the ions and the smaller the distance between the ions, the stronger the attraction and the higher the lattice energy. Although the charges of Mg+2 and Sr+2 are the same, Sr is a much larger ion due to its greater amount of occupied energy shells. Since it is larger, the distance between Sr+2 and the chlorine ions is greater than the distance between Mg+2 and the chlorine ions. Therefore, the lattice energy of SrCl2 (s) must be less than 2300 kJ/mol.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

© 2023 Fiveable Inc. All rights reserved.